Posts tagged ‘FDA’
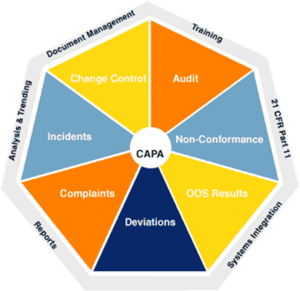
Most manufacturing sites these days have CAPA systems – a mechanism to capture, record, control and approve or reject Corrective Actions. Despite the fact that a lot of focus has been given to CAPA in recent years, there is a great deal of misunderstanding and a clear lack of clarity on what CAPA actually is. This may explain why many organisations feel that they are still in “fire-fighting” mode – always dealing with yesterday’s problems rather than preventing future issues from occurring. Read more